rfid chip breast implant Chip technology marks the spot for breast biopsies. Radio frequency . Ready to declutter your wallet? In this comprehensive Apple Wallet tutorial, we'll show you how to add any card to your Apple Wallet, from loyalty and member.
0 · rfid implants before and after
1 · rfid chips in humans
2 · rfid chip implant near me
3 · rfid chip implant law 2020
4 · rfid chip implant law
5 · how to remove microchip implant
6 · how to disable rfid implant
7 · dangers of microchipping humans
A contactless credit card uses RFID technology to enable you to hover or tap a card over a card terminal as a means of conducting a transaction. The card emits short-range . See more
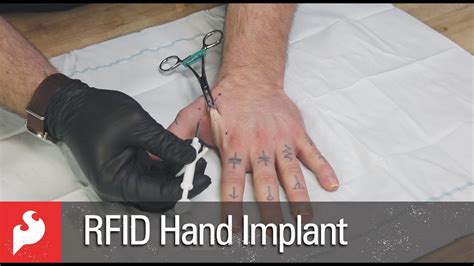
rfid implants before and after
In our opinion, breast implants with RFID chips should be avoided until results from more research are available. We repeat the request to researchers, clinicians, and industry to publish their experiences, and ideally develop identification chips with reduced magnetic forces. Chip technology marks the spot for breast biopsies. Radio frequency . We raise the question if the benefits of RFID-labeled silicone implants outweigh .Although the RFID-M is considered compatible with magnetic resonance imaging (MRI), the .
The RFID technology behind the Ergonomix implant was provided by VeriTeQ .• LOCalizer is a wireless radiofrequency identification (RFID) breast lesion localization system. . Use of Hologic LOCalizer radiofrequency identification (RFID) tags to localise .
SmoothSilk implants (SSI) are the first generation of implants to incorporate a .MRI artifacts caused by the RFID-M system in SSI gel implants in patients who underwent . We examined a patient with silicone implants containing RFID chips with . In our opinion, breast implants with RFID chips should be avoided until results from more research are available. We repeat the request to researchers, clinicians, and industry to publish their experiences, and ideally develop identification chips with reduced magnetic forces.
Chip technology marks the spot for breast biopsies. Radio frequency identification, also called RFID, is a technology that uses wireless radio waves to transfer data and identify objects. It’s used in many ways, from inventory tracking and race timing to dairy herd management and car rental returns. We raise the question if the benefits of RFID-labeled silicone implants outweigh the drawbacks of magnetic resonance artifacts caused by the RFID chip itself. Patients with silicone filled breast implants will likely need radiological breast .Although the RFID-M is considered compatible with magnetic resonance imaging (MRI), the size of the artifact and its influence on breast tissue vary. This prospective study assessed safety and MRI issues in a cohort of breast reconstruction patients.
The RFID technology behind the Ergonomix implant was provided by VeriTeQ Corporation. Known as Q Inside Safety Technology, the system consists of an FDA-approved implantable transponder chip, a proprietary handheld reader, and a secure online database.• LOCalizer is a wireless radiofrequency identification (RFID) breast lesion localization system. It uses a RFID chip and a handheld reader with probe. The RFID is designed to be implanted in the lesion up to 30 days before surgery and is free of radioactivity. It was awarded 510k clearance by the FDA on 5/1/17 . 5.
Use of Hologic LOCalizer radiofrequency identification (RFID) tags to localise impalpable breast lesions and axillary nodes: experience of the first 150 cases in a UK breast unit. Lowes S, Bell A, Milligan R, Amonkar S, Leaver A. Clin .
SmoothSilk implants (SSI) are the first generation of implants to incorporate a radio-frequency identification device (RFID-M), a non-invasive traceability.

MRI artifacts caused by the RFID-M system in SSI gel implants in patients who underwent breast reconstruction. Material and Methods This prospective, single-center study involved all consec-utive breast reconstructions using SSI (Motiva/Establish-ment Labs, Coyol Free Zone, Alajuela, Costa Rica). All
We examined a patient with silicone implants containing RFID chips with magnetic resonance imaging and were surprised by the artifacts caused by the RFID chip. In our opinion, breast implants with RFID chips should be avoided until results from more research are available. We repeat the request to researchers, clinicians, and industry to publish their experiences, and ideally develop identification chips with reduced magnetic forces. Chip technology marks the spot for breast biopsies. Radio frequency identification, also called RFID, is a technology that uses wireless radio waves to transfer data and identify objects. It’s used in many ways, from inventory tracking and race timing to dairy herd management and car rental returns. We raise the question if the benefits of RFID-labeled silicone implants outweigh the drawbacks of magnetic resonance artifacts caused by the RFID chip itself. Patients with silicone filled breast implants will likely need radiological breast .
Although the RFID-M is considered compatible with magnetic resonance imaging (MRI), the size of the artifact and its influence on breast tissue vary. This prospective study assessed safety and MRI issues in a cohort of breast reconstruction patients.
rfid chips in humans
The RFID technology behind the Ergonomix implant was provided by VeriTeQ Corporation. Known as Q Inside Safety Technology, the system consists of an FDA-approved implantable transponder chip, a proprietary handheld reader, and a secure online database.• LOCalizer is a wireless radiofrequency identification (RFID) breast lesion localization system. It uses a RFID chip and a handheld reader with probe. The RFID is designed to be implanted in the lesion up to 30 days before surgery and is free of radioactivity. It was awarded 510k clearance by the FDA on 5/1/17 . 5.
Use of Hologic LOCalizer radiofrequency identification (RFID) tags to localise impalpable breast lesions and axillary nodes: experience of the first 150 cases in a UK breast unit. Lowes S, Bell A, Milligan R, Amonkar S, Leaver A. Clin . SmoothSilk implants (SSI) are the first generation of implants to incorporate a radio-frequency identification device (RFID-M), a non-invasive traceability.MRI artifacts caused by the RFID-M system in SSI gel implants in patients who underwent breast reconstruction. Material and Methods This prospective, single-center study involved all consec-utive breast reconstructions using SSI (Motiva/Establish-ment Labs, Coyol Free Zone, Alajuela, Costa Rica). All

walmart smart talk phone cards
Today’s cards typically use a version of RFID called near-field communication, or NFC, which operates at a higher frequency and allows for faster data transfer, .
rfid chip breast implant|rfid chip implant law 2020